The Science Behind Drug Dissolution
What is the Noyes-Whitney Equation?
The Noyes-Whitney equation describes the rate at which a solid substance dissolves in a solvent under static conditions. It’s particularly significant for oral solid dosage forms like tablets and capsules.
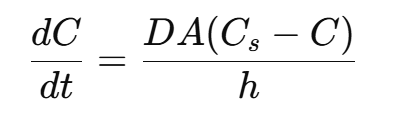

Why Noyes-Whitney Equation Matters in Pharmaceutical Sciences?
1. Dissolution → Bioavailability
In oral drug delivery, the drug must first dissolve in gastrointestinal fluids before it can be absorbed. Poor dissolution leads to low or erratic absorption—especially in BCS Class II and IV drugs (low solubility).
2. Preformulation Strategy
Formulators manipulate D, A, and h to enhance dissolution:
- Reducing particle size (↑ A)
- Using surfactants or wetting agents (↓ h)
- Selecting amorphous over crystalline forms (↑ Cₛ)
3. Predictive Tool for In-Vitro Testing
Dissolution testing using USP Apparatus (1 & 2) is guided by this principle. It is used to:
- Predict in-vivo behavior via IVIVC (In-Vitro In-Vivo Correlation)
- Compare different formulations
- Justify biowaivers
Practical Implications of Noyes-Whitney Equation in Formulation
| Parameter | How It Affects Dissolution | Pharma Strategy |
|---|---|---|
| Surface Area (A) | ↑ A → Faster dissolution | Micronization, nanosizing |
| Diffusion Coefficient (D) | ↑ D → Faster diffusion | Use of co-solvents, temperature control |
| Saturation Solubility (Cₛ) | ↑ Cₛ → Greater driving force | Salt formation, amorphous drugs |
| Boundary Layer Thickness (h) | ↓ h → Reduced resistance | Surfactants, stirring |
Limitations and Modern Adaptations of Noyes-Whitney Equation
While the Noyes-Whitney equation provides a first-order approximation, it assumes:
- A stagnant diffusion layer
- Constant surface area
- No solid-solvent interactions
Modern extensions like the Nernst-Brunner equation and diffusion layer models incorporate variable surface area and dynamic fluid interactions for greater accuracy in controlled-release formulations and nanoparticle suspensions.
Relevance of Noyes-Whitney Equation to BCS and Regulatory Frameworks
Regulatory bodies like the USFDA and EMA rely on dissolution data to:
- Grant biowaivers
- Evaluate generic equivalence
- Approve controlled release mechanisms
The Noyes-Whitney framework is embedded in BCS-based formulation design and QbD (Quality by Design) approaches.
The Noyes-Whitney equation, despite its simplicity, remains a cornerstone of pharmaceutical science. Whether you’re optimizing a poorly soluble API, designing a sustained-release tablet, or scaling up your dissolution testing protocol, this equation serves as a reliable compass guiding science-backed formulation decisions.
In the era of QbD, personalized medicine, and predictive modeling, the relevance of foundational science cannot be overstated. The Noyes-Whitney equation reminds us that great science is not about complexity—it’s about clarity. And when it comes to ensuring that every milligram of medicine counts, that clarity can make all the difference.
Source – Noyes-Whitney framework






